Next: Atomic Spectra * Up: The Problems with Classical Previous: The Photoelectric Effect Contents
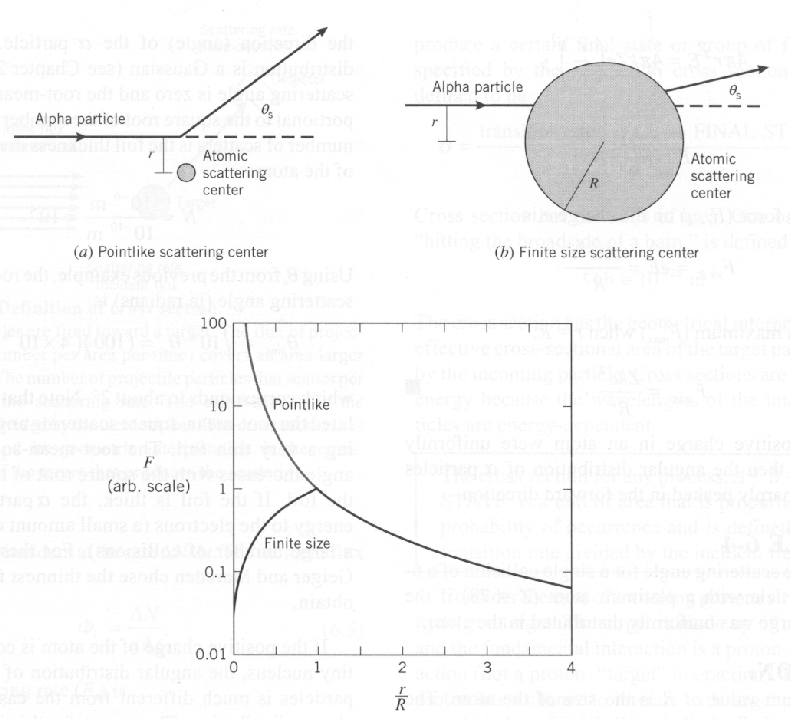
He found that the
![]() particles often scattered at angles larger than 90 degrees.
His data can be explained if the
positive nucleus
of an atom is very small.
For a very small nucleus, the Coulomb force continues to increase as the
particles often scattered at angles larger than 90 degrees.
His data can be explained if the
positive nucleus
of an atom is very small.
For a very small nucleus, the Coulomb force continues to increase as the
![]() approaches the nucleus, and backscattering is possible.
approaches the nucleus, and backscattering is possible.

* Example:
Use Electrostatics to estimate how small a gold nucleus must be to
backscatter a 5.5 MeV alpha particle.*
This brought up a new problem. The atomic size was known from several types of experiments. If electrons orbit around the atomic nucleus, according to Maxwell's equations, they should radiate energy as they accelerate. This radiation is not observed and the ground states of atoms are stable.
In Quantum Mechanics, the localization of the electron around a nucleus is limited because of the wave nature of the electron. For hydrogen, where there is no multi-body problem to make the calculation needlessly difficult, the energy levels can be calculated very accurately. Hydrogen was used to test Quantum Mechanics as it developed. We will also use hydrogen a great deal in this course.
Scattering of the high energy
![]() particles allowed Rutherford to
``see'' inside the atom
and determine that the atomic nucleus is very small.
(He probably destroyed all the atoms he ``saw''.)
The figure below shows Rutherford's angular distribution in his scattering experiment
along with several subsequent uses of the same technique, with higher and higher energy particles.
We see Rutherford's
discovery of the tiny nucleus, the discovery of nuclear structure, the discovery
of a point-like proton inside the nucleus, the discovery of proton structure, the discovery of
quarks inside the proton, and finally the lack of discovery, so far, of any quark structure.
particles allowed Rutherford to
``see'' inside the atom
and determine that the atomic nucleus is very small.
(He probably destroyed all the atoms he ``saw''.)
The figure below shows Rutherford's angular distribution in his scattering experiment
along with several subsequent uses of the same technique, with higher and higher energy particles.
We see Rutherford's
discovery of the tiny nucleus, the discovery of nuclear structure, the discovery
of a point-like proton inside the nucleus, the discovery of proton structure, the discovery of
quarks inside the proton, and finally the lack of discovery, so far, of any quark structure.
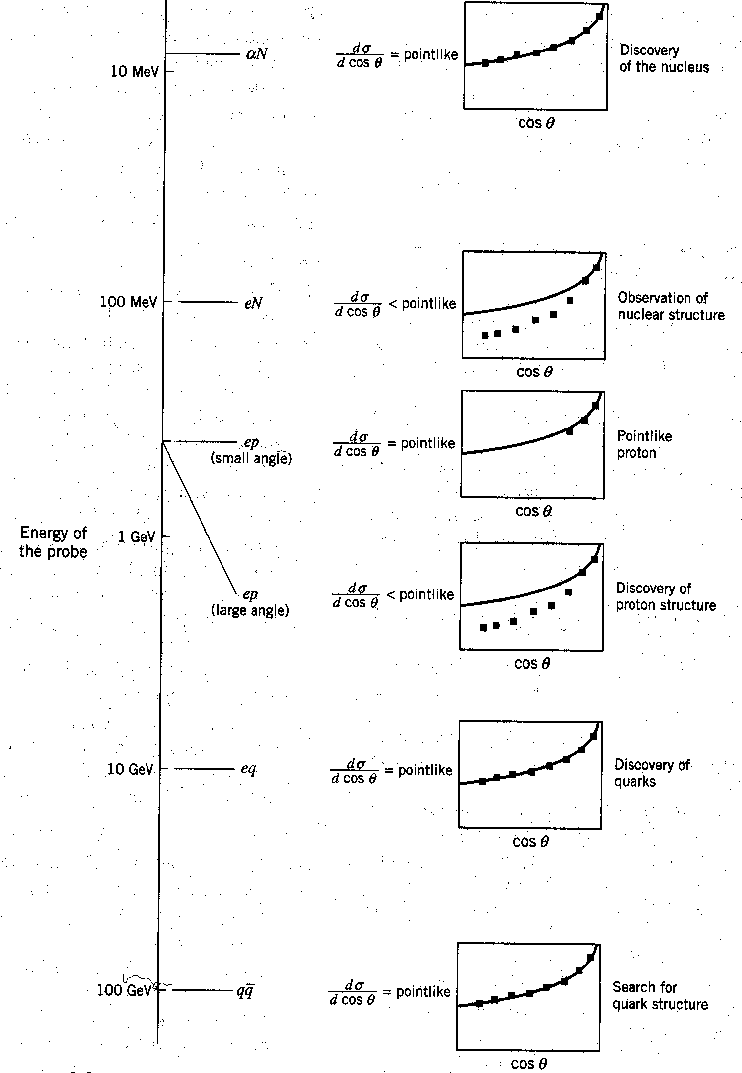
To ``see'' these things at smaller and smaller distances, we needed to use beams of particles with smaller and smaller wavelength, and hence, higher energy.
Jim Branson 2013-04-22