Next: Rotational States Up: Molecular Physics Previous: Molecular Orbitals Contents
We can estimate the energy of the vibrational levels.
If
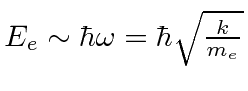 , then crudely the proton has the same spring constant
, then crudely the proton has the same spring constant
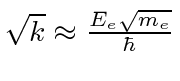 .
.
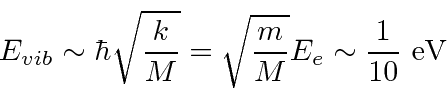
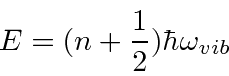
Complex molecules can have many different modes of vibration. Diatomic molecules have just one.
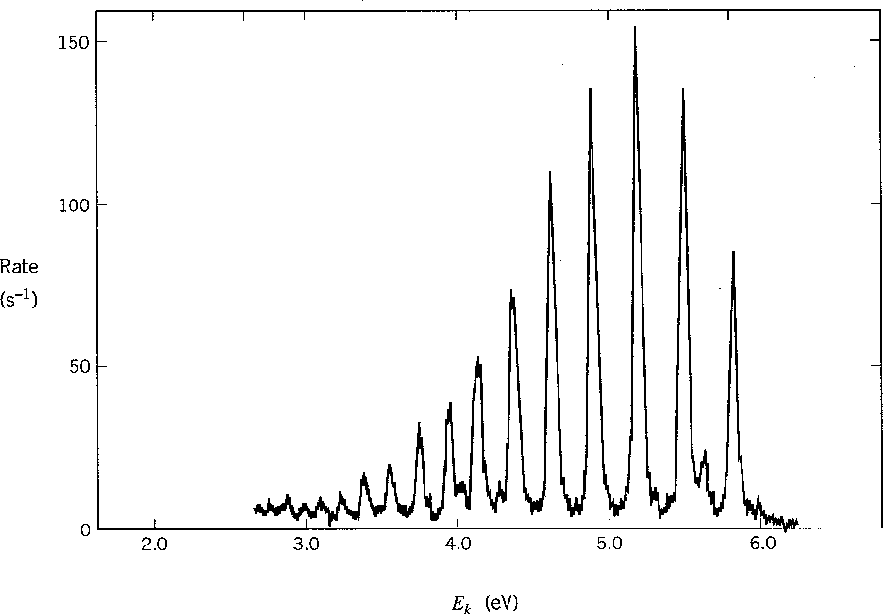
The graph below shows the energy spectrum of electrons knocked out of molecular
hydrogen by UV photons (photoelectric effect).
The different peaks correspond to the vibrational state of the final H![]() ion.
ion.
Can you calculate the number of vibrational modes for a molecule compose of 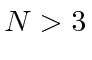 atoms.
atoms.
Jim Branson 2013-04-22