Next: General Addition of Angular Up: Addition of Angular Momentum Previous: Adding Spin to Integer Contents
A common way to name states in atomic physics is to use spectroscopic notation.
It is essentially a standard way to write down the angular momementum quantum numbers of
a state.
The general form is
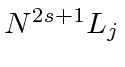 , where
, where
![]() is the principal quantum number
and will often be omitted,
is the principal quantum number
and will often be omitted,
![]() is the total spin quantum number (
is the total spin quantum number (
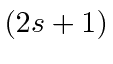 is the number of spin states),
is the number of spin states),
![]() refers to the orbital angular momentum quantum number
refers to the orbital angular momentum quantum number
![]() but is written
as
but is written
as
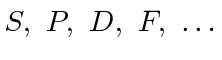 for
for
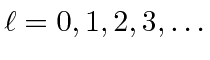 ,
and
,
and
![]() is the total angular momentum quantum number.
is the total angular momentum quantum number.
A quick example is the single electron states, as we find in Hydrogen. These are:
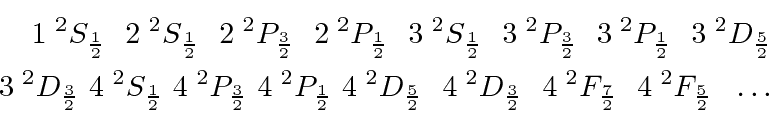
For atoms with more than one electron, the total spin state has more possibilities and perhaps several ways to make a state with the same quantum numbers.
Jim Branson 2013-04-22