Next: Carbon Ground State Up: Examples Previous: Examples Contents
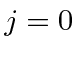 as do all closed shells.
The valence electron is in the 2P state and hence has
as do all closed shells.
The valence electron is in the 2P state and hence has
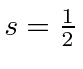 .
Since the shell is not half full we couple to the the lowest
.
Since the shell is not half full we couple to the the lowest
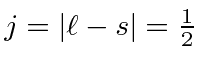 .
So the ground state is
.
So the ground state is
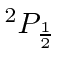 .
.
|
|
e |
| 1 |
|
| 0 | |
| -1 | |
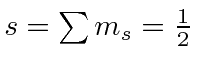 |
|
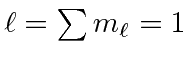 |
|