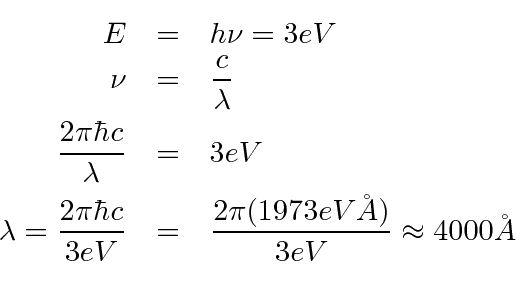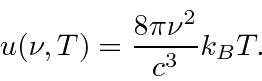- What is the maximum wavelength of electromagnetic radiation
which can eject electrons from a metal having a work function of 3 eV?
(3 points)
Answer
The minimum photon energy needed to knock out an electron is 3 eV.
We just need to convert that to wavelength.
- *
Based on classical electromagnetism and statistical mechanics, Rayleigh
computed the energy density inside a cavity. He found that, at a
temperature T, the energy density as a function of frequency was
Why is this related to black body radiation? Why was this in obvious
disagreement with observation?
- What is the maximum wavelength of electromagnetic radiation
which can eject electrons from a metal having a work function of 2 eV?
- *
State simply what problem with black-body radiation
caused Plank to propose the relation
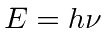 for light.
for light.
- The work function of a metal is 2 eV.
Assume a beam of light of wavelength
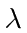 is incident upon a polished
surface of the metal.
Plot the maximum electron energy (in eV) of electrons ejected from the metal
versus
is incident upon a polished
surface of the metal.
Plot the maximum electron energy (in eV) of electrons ejected from the metal
versus 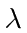 in Angstroms.
Be sure to label both axes with some numerical values.
in Angstroms.
Be sure to label both axes with some numerical values.
Jim Branson
2013-04-22