Next: Examples Up: Atomic Physics Previous: The Periodic Table Contents
Even with the strong nuclear force, a shell model describes important features
of nuclei.
Nuclei have tightly bound closed shells for both protons and neutrons.
Tightly bound nuclei correspond to the most abundant elements.
What elements exist is governed by nuclear physics and we can get a good idea
from a simple shell model.
Nuclear magic numbers occur for neutron or proton number of
2, 8, 20, 28, 50, 82, and 126, as indicated in the figure below.
Nuclei where the number of protons or neutrons is magic are more tightly bound
and often more abundant.
Heavier nuclei tend to have more neutrons than protons because of the coulomb
repulsion of the protons (and the otherwise symmetric strong interactions).
Nuclei which are doubly magic are very tightly bound compared to neighboring
nuclei.
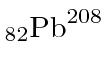 is a good example of a doubly magic nucleus with many more neutrons than
protons.
is a good example of a doubly magic nucleus with many more neutrons than
protons.
Remember, its only hydrogen states which are labeled with a principle quantum number
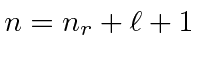 .
In the nuclear shell model,
.
In the nuclear shell model,
![]() refers only to the radial excitation
so states like the
refers only to the radial excitation
so states like the
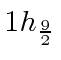 show up in real nuclei and on the following chart.
The other feature of note in the nuclear shell model is that the nuclear
spin orbit interaction is strong and of the opposite sign to that in atoms.
The splitting between states of different
show up in real nuclei and on the following chart.
The other feature of note in the nuclear shell model is that the nuclear
spin orbit interaction is strong and of the opposite sign to that in atoms.
The splitting between states of different
![]() is smaller than that but of the same order as splitting
between radial or angular excitations.
It is this effect and the shell model for which Maria Mayer got her Nobel prize.
is smaller than that but of the same order as splitting
between radial or angular excitations.
It is this effect and the shell model for which Maria Mayer got her Nobel prize.
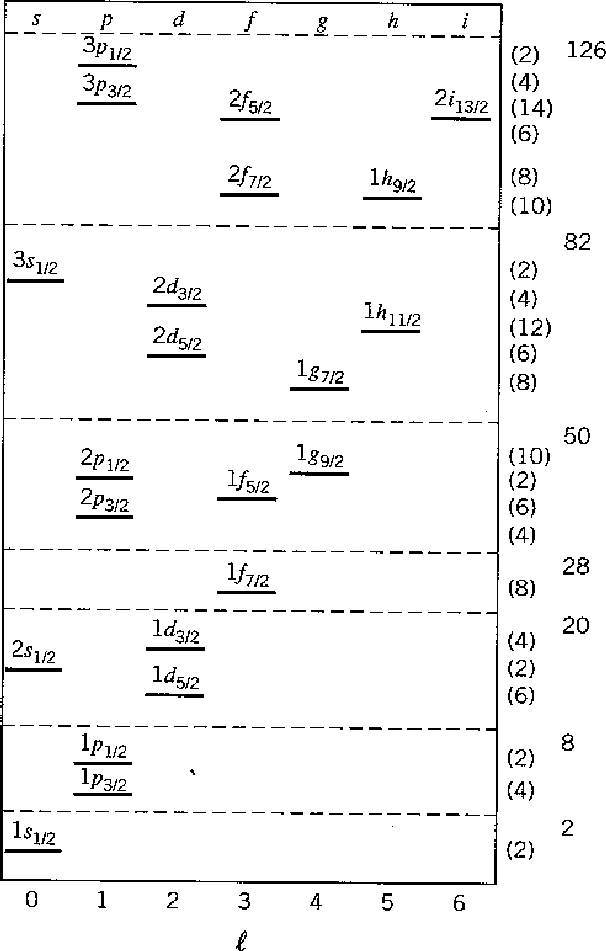
Another feature of nuclei not shown in the table is that the spin-spin force very much favors nucleons which are paired. So nuclear isotopes with odd numbers of protons or odd numbers of neutrons have less binding energy and nuclei with odd numbers of both protons and neutrons are unstable (with one exception).
Jim Branson 2013-04-22