Next: The Neutral Kaon System* Up: Other Two State Systems* Previous: Other Two State Systems* Contents
Since the Nitrogen atom can tunnel from one side of the molecule to the other, there are cross terms in the
Hamiltonian (limiting ourselves to the two symmetric ground states).
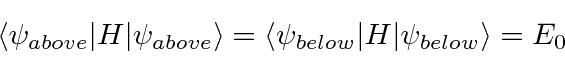
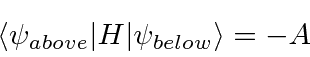
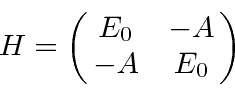
The energy eigenvalues can be found from the usual equation.
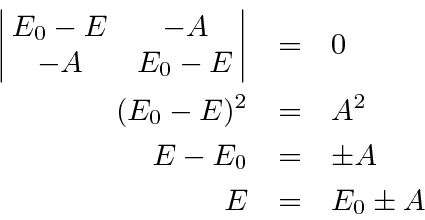
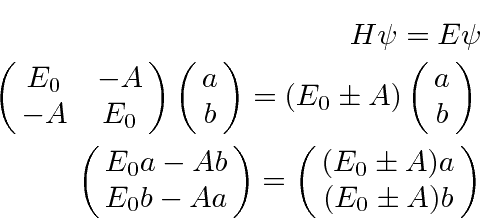
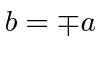 .
Substituting auspiciously, we get.
.
Substituting auspiciously, we get.
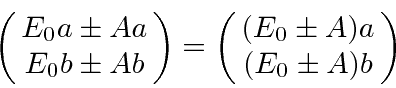
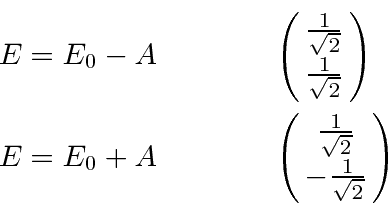
Feynman goes on to further split the states by putting the molecules in an electric field. This makes the diagonal terms of the Hamiltonian slightly different, like a magnetic field does in the case of spin.
Finally, Feynman studies the effect of Ammonia in an oscillating Electric field, the Ammonia Maser.
Jim Branson 2013-04-22