Next: Can I ``See'' inside Up: Examples Previous: The Dirac Delta Function Contents
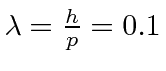 Å.
Å.
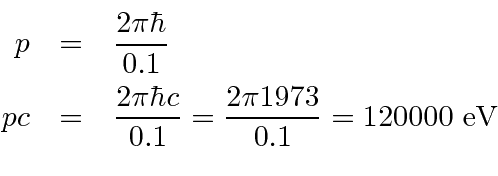
A similar calculation can be made with the uncertainty principle.
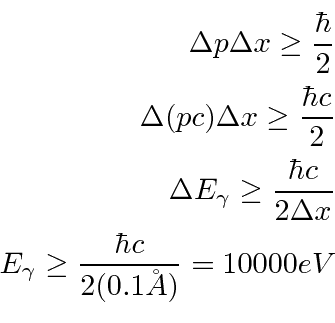
So we can't ``watch'' the inside of an atom.
We can probe atoms with high energy photons (for example). These will blow the atoms apart, but we can use many atoms of the same kind. We learn about the internal structure of the atoms by scattering particles off them, blowing them apart.
Jim Branson 2013-04-22