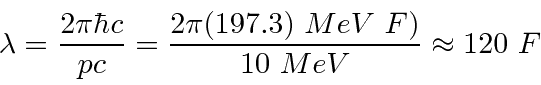- What is the deBroglie wavelength of an electron with 13.6 eV of kinetic energy?
What is the deBroglie wavelength of an electron with 10 MeV of kinetic energy?
Answer
13.6 eV is much less than
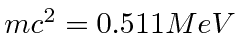 so this is non-relativistic.
so this is non-relativistic.
10 MeV is much bigger than 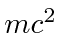 for an electron so it is super-relativistic and we can use
for an electron so it is super-relativistic and we can use 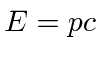 .
.
- What is the DeBroglie wavelength for each of the following particles?
The energies given are the kinetic energies.
- a)
- a 10 eV electron
- b)
- a 1 MeV electron
- c)
- a 10 MeV proton
- A 2 slit electron diffraction experiment is set up as (not) shown below.
The observed electron intensity distribution is plotted in the figure. Now
an intense light source is introduced near the two slits. With this light,
it can be "seen" which slit each electron goes through. Qualitatively plot
the new electron intensity distribution from each slit and from the 2 slits
combined. What is the condition on the wavelength of the light for
this effect to occur?
- What is the DeBroglie wavelength for each of the following particles?
The energies given are the kinetic energies.
- a)
- a 1 eV electron
- b)
- a
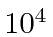 MeV proton
MeV proton
- What K.E. must a Hydrogen atom have so that its DeBroglie wavelength
is smaller than the size of the atom? (Factors of 2 are not important.)
- Calculate the DeBroglie wavelength for (a) a proton with
10 MeV kinetic energy, (b) An electron with 10 MeV kinetic energy, and
(c) a 1 gram lead ball moving with a velocity of 10 cm/sec (one erg
is one gram cm
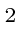 /sec
/sec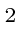 ). Be sure to take account of relativity
where needed.
). Be sure to take account of relativity
where needed.
Jim Branson
2013-04-22
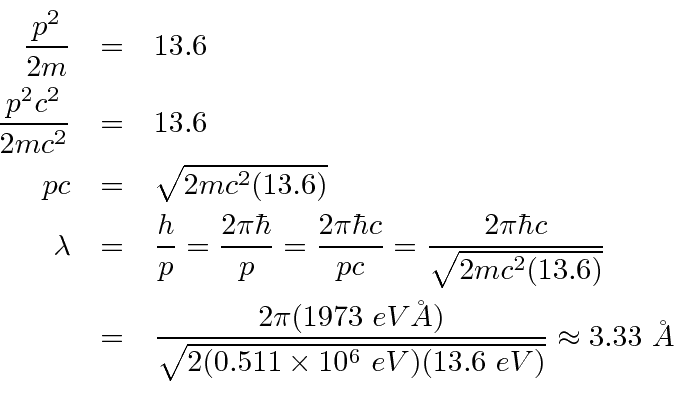
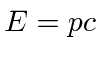 .
.