- List the possible spectroscopic states that can arise in the following
electronic configurations:
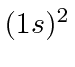 ,
, 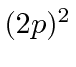 ,
, 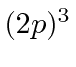 ,
, 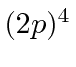 , and
, and 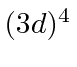 .
Take the exclusion principle into account. Which should be the ground state?
.
Take the exclusion principle into account. Which should be the ground state?
- Use Hund's rules to find the spectroscopic description of the
ground states of the following atoms: N(
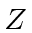 =7), K(
=7), K(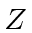 =19), Sc(
=19), Sc(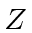 =21),
Co(
=21),
Co(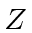 =27). Also determine the electronic configuration.
=27). Also determine the electronic configuration.
- Use Hund's rules to check the
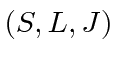 quantum numbers of the elements
with
quantum numbers of the elements
with 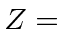 14, 15, 24, 30, 34.
14, 15, 24, 30, 34.
Jim Branson
2013-04-22
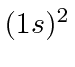 ,
, 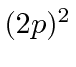 ,
, 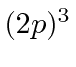 ,
, 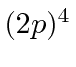 , and
, and 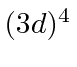 .
Take the exclusion principle into account. Which should be the ground state?
.
Take the exclusion principle into account. Which should be the ground state?
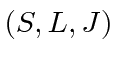 quantum numbers of the elements
with
quantum numbers of the elements
with