Next: The Nuclear Shell Model Up: Atomic Physics Previous: Hund's Rules Contents
| Z | El. | Electron Configuration |
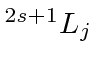 |
Ioniz. Pot. |
| 1 | H |
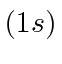 |
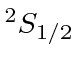 |
13.6 |
| 2 | He |
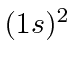 |
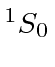 |
24.6 |
| 3 | Li | He
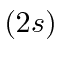 |
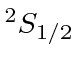 |
5.4 |
| 4 | Be | He
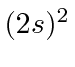 |
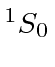 |
9.3 |
| 5 | B | He
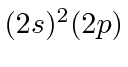 |
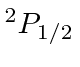 |
8.3 |
| 6 | C | He
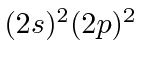 |
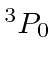 |
11.3 |
| 7 | N | He
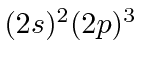 |
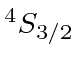 |
14.5 |
| 8 | O | He
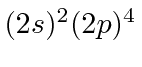 |
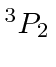 |
13.6 |
| 9 | F | He
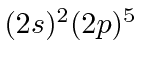 |
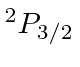 |
17.4 |
| 10 | Ne | He
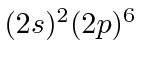 |
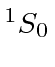 |
21.6 |
| 11 | Na | Ne
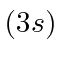 |
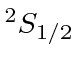 |
5.1 |
| 12 | Mg | Ne
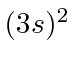 |
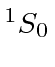 |
7.6 |
| 13 | Al | Ne
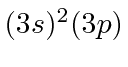 |
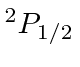 |
6.0 |
| 14 | Si | Ne
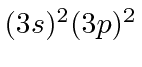 |
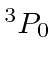 |
8.1 |
| 15 | P | Ne
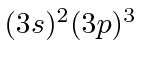 |
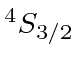 |
11.0 |
| 16 | S | Ne
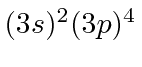 |
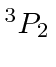 |
10.4 |
| 17 | Cl | Ne
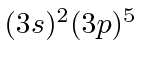 |
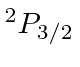 |
13.0 |
| 18 | Ar | Ne
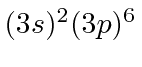 |
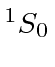 |
15.8 |
| 19 | K | Ar
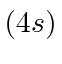 |
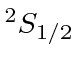 |
4.3 |
| 20 | Ca | Ar
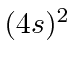 |
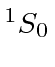 |
6.1 |
| 21 | Sc | Ar
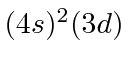 |
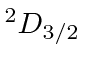 |
6.5 |
| 22 | Ti | Ar
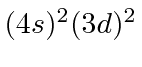 |
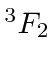 |
6.8 |
| 23 | V | Ar
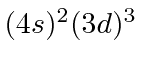 |
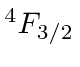 |
6.7 |
| 24 | Cr | Ar
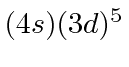 |
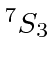 |
6.7 |
| 25 | Mn | Ar
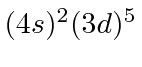 |
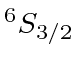 |
7.4 |
| 26 | Fe | Ar
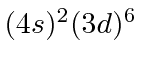 |
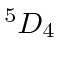 |
7.9 |
| 36 | Kr | (Ar)
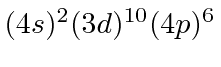 |
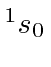 |
14.0 |
| 54 | Xe | (Kr)
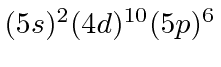 |
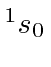 |
12.1 |
| 86 | Rn | (Xe)
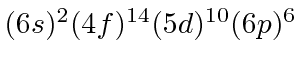 |
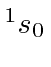 |
10.7 |
We see that the atomic shells fill up in the order 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p.
The effect of screening increasing the energy of higher
![]() states is clear.
Its no wonder that the periodic table is not completely periodic.
states is clear.
Its no wonder that the periodic table is not completely periodic.
The Ionization Potential column gives the energy in eV needed to remove one electron from the atom, essentially the Binding energy of the last electron. The Ionization Potential peaks for atoms with closed shells, as the elctron gains binding energy from more positive charge in the the nucleus without much penalty from repulsion of the other electrons in the shell. As charge is added to the nucleus, the atom shrinks in size and becomes more tightly bound. A single electron outside a closed shell often has the lowest Ionization Potential because it is well screened by the inner electrons. The figure below shows a plot of ionization potential versus Z.
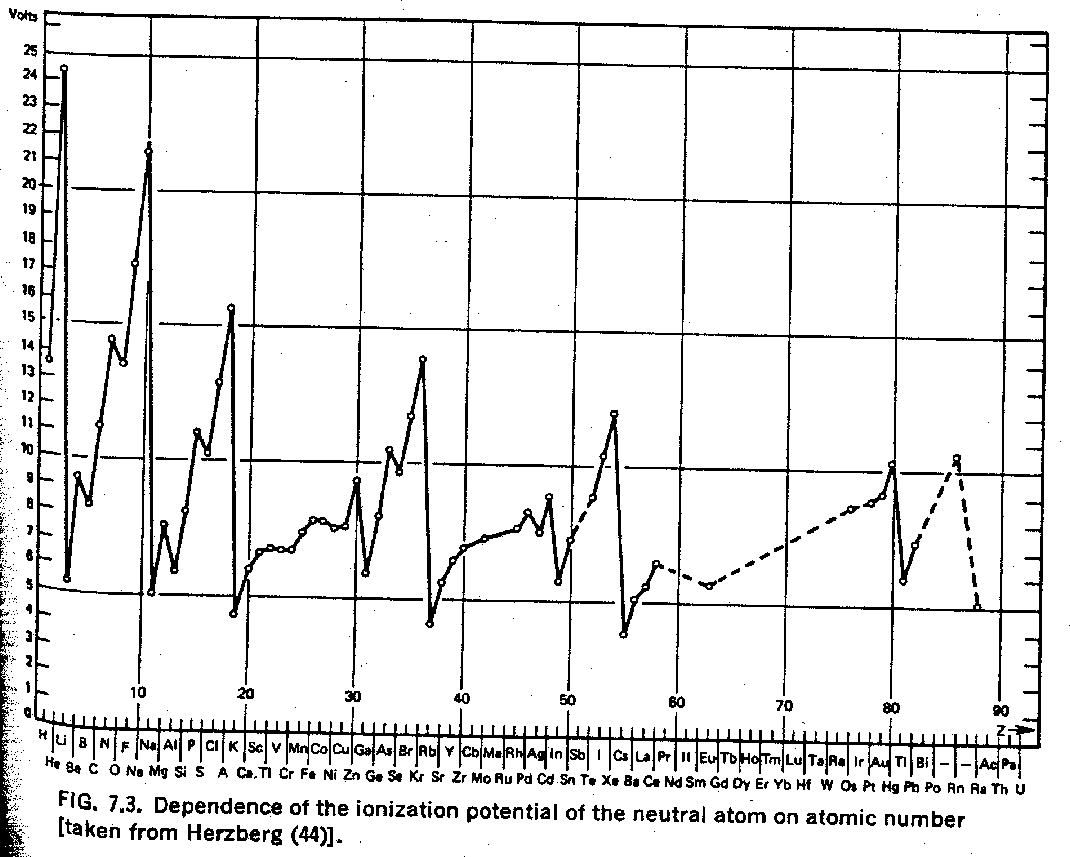
Jim Branson 2013-04-22