Next: Hyperfine Splitting in a Up: Examples Previous: Examples Contents
The ground state of Hydrogen has a spin
![]() electron coupled to a spin
electron coupled to a spin
![]() proton, giving total angular momentum state of
proton, giving total angular momentum state of
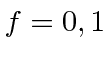 .
We have computed in first order perturbation theory that
.
We have computed in first order perturbation theory that
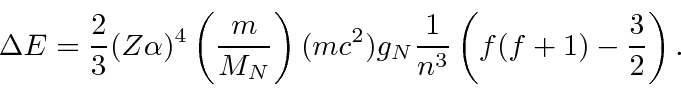
The energy difference between the two hyperfine levels determines the wave length of the radiation emitted in hyperfine transitions.
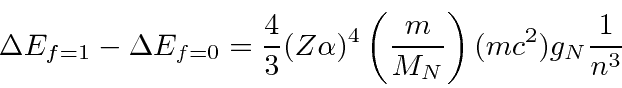
For
![]() Hydrogen, this gives
Hydrogen, this gives
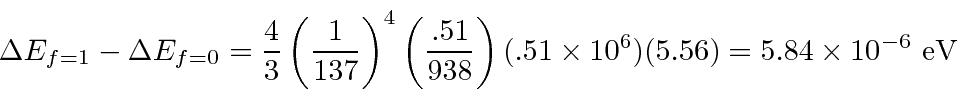
Recall that at room temperature,
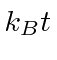 is about
is about
![]() eV, so the states
have about equal population at room temperature.
Even at a few degrees Kelvin, the upper state is populated so that transitions
are possible.
The wavelength is well known.
eV, so the states
have about equal population at room temperature.
Even at a few degrees Kelvin, the upper state is populated so that transitions
are possible.
The wavelength is well known.
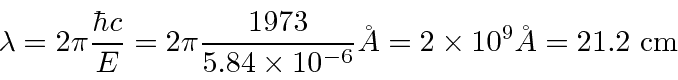
 state is excited by collisions.
Electromagnetic transitions are slow because of the selection rule
state is excited by collisions.
Electromagnetic transitions are slow because of the selection rule
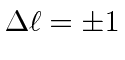 we will learn later, and because of the small energy difference.
The
we will learn later, and because of the small energy difference.
The
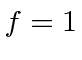 state does emit a photon to de-excite and those photons have a long
mean free path in the gas.
state does emit a photon to de-excite and those photons have a long
mean free path in the gas.
Jim Branson 2013-04-22