Next: Atomic Physics Up: Course Summary Previous: Hyperfine Structure Contents
The Hamiltonian for
Helium
has the same terms as Hydrogen but has a large
perturbation due to the repulsion between the two electrons.
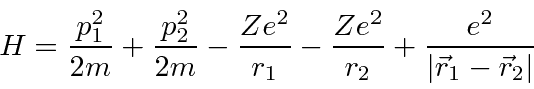
The Helium ground state has two electrons in the 1s level.
Since the spatial state is symmetric, the spin part of the state must be antisymmetric so
![]() (as it always is for closed shells).
For our zeroth order energy eigenstates, we will use
product states of Hydrogen wavefunctions
(as it always is for closed shells).
For our zeroth order energy eigenstates, we will use
product states of Hydrogen wavefunctions
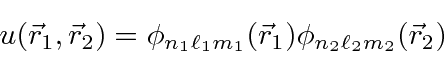
We can estimate the ground state energy in first order perturbation theory, using the electron repulsion term as a (very large) perturbation. This is not very accurate.
We can improve the estimate of the ground state energy using the variational principle.
The main problem with our estimate from perturbation theory is that we are not accounting for
changes in the wave function of the electrons due to screening.
We can do this in some reasonable approximation by reducing the charge of the nucleus in the wavefunction
(not in the Hamiltonian).
With the parameter
![]() , we get a better estimate of the energy.
, we get a better estimate of the energy.
| Calculation | Energy |
 |
|
|
-108.8 | 2 |
|
|
-74.8 | 2 |
|
|
-77.38 |
|
| Actual | -78.975 |
There is only one allowed
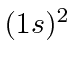 state and it is the ground state.
For excited states, the spatial states are (usually) different so
they can be either symmetric or antisymmetric (under interchange of the two electrons).
It turns out that the antisymmetric state has the electrons further apart so the repulsion is smaller
and the energy is lower.
If the spatial state is antisymmetric, then the spin state is symmetric, s=1.
So the triplet states are generally significantly lower in energy than the corresponding spin singlet states.
This
appears to be a strong spin dependent interaction but is actually just the effect of the repulsion between the electrons
having a big effect depending on the symmetry of the spatial state and hence on the symmetry of the spin state.
state and it is the ground state.
For excited states, the spatial states are (usually) different so
they can be either symmetric or antisymmetric (under interchange of the two electrons).
It turns out that the antisymmetric state has the electrons further apart so the repulsion is smaller
and the energy is lower.
If the spatial state is antisymmetric, then the spin state is symmetric, s=1.
So the triplet states are generally significantly lower in energy than the corresponding spin singlet states.
This
appears to be a strong spin dependent interaction but is actually just the effect of the repulsion between the electrons
having a big effect depending on the symmetry of the spatial state and hence on the symmetry of the spin state.
The first exited state has the hydrogenic state content of (1s)(2s) and has s=1. We calculated the energy of this state.
We'll learn later that electromagnetic transitions which change spin are strongly suppressedcausing the spin triplet (orthohelium) and the spin singlet states (parahelium) to have nearly separate decay chains.
Jim Branson 2013-04-22