Next: Derivations and Calculations Up: Hydrogen Previous: The Radial Wavefunction Solutions Contents
The ground state of Hydrogen has
![]() and
and
![]() .
This is conventionally called the 1s state.
The convention is to name
.
This is conventionally called the 1s state.
The convention is to name
![]() states ``s'',
states ``s'',
![]() states ``p'',
states ``p'',
![]() states ``d'', and
states ``d'', and
![]() states ``f''.
From there on follow the alphabet with g, h, i, ...
states ``f''.
From there on follow the alphabet with g, h, i, ...
The first excited state of Hydrogen has
![]() .
There are actually four degenerate states (not counting different spin states) for
.
There are actually four degenerate states (not counting different spin states) for
![]() .
In terms of
.
In terms of
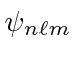 , these are
, these are
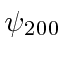 ,
,
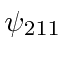 ,
,
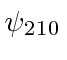 , and
, and
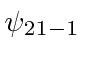 .
These would be called the 2s and 2p states.
Remember, all values of
.
These would be called the 2s and 2p states.
Remember, all values of
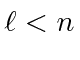 are allowed.
are allowed.
The second excited state has
![]() with the 3s, 3p and 3d states being degenerate.
This totals 9 states with the different allowed
with the 3s, 3p and 3d states being degenerate.
This totals 9 states with the different allowed
![]() values.
values.
In general there are
![]() degenerate states, again not counting different spin states.
degenerate states, again not counting different spin states.
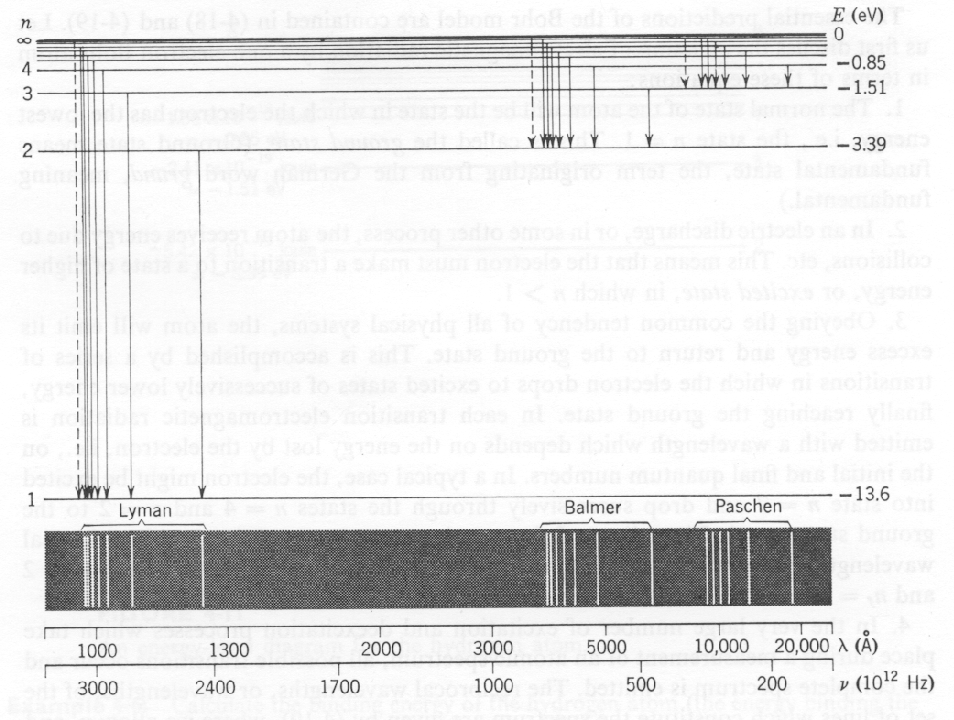
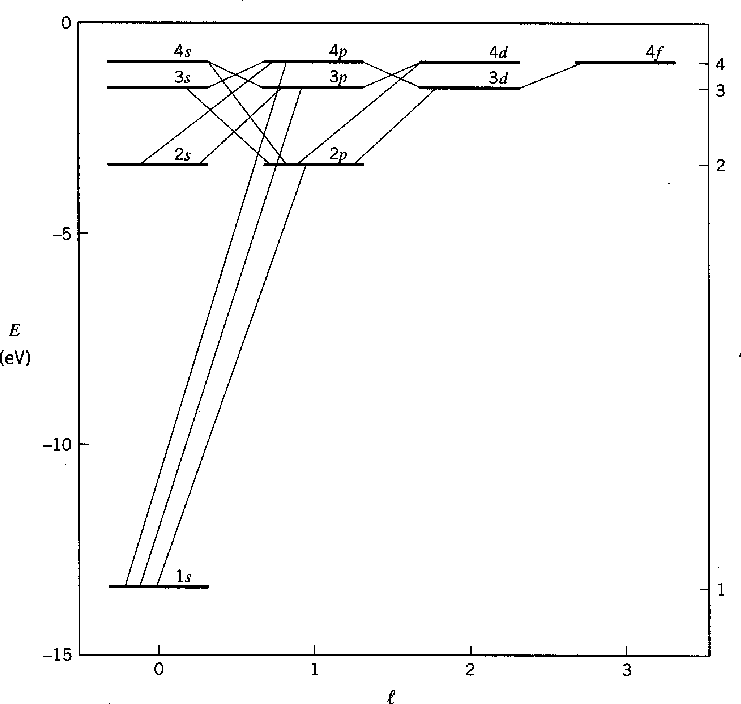
The Hydrogen spectrum was primarily investigated by measuring the energy of photons emitted in transitions between the states, as depicted in the figures above and below.
Jim Branson 2013-04-22