Next: The Radial Wavefunction Solutions Up: Quantum Physics 130 Previous: Sample Test Problems Contents
The Hydrogen atom consists of an electron bound to a proton by the Coulomb potential.
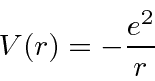
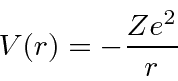
Since the potential is spherically symmetric, the problem separates and the solutions
will be a product of a radial wavefunction and one of the spherical harmonics.
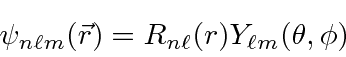
The radial wavefunction satisfies the differential equation that depends on the angular
momentum quantum number
![]() ,
,
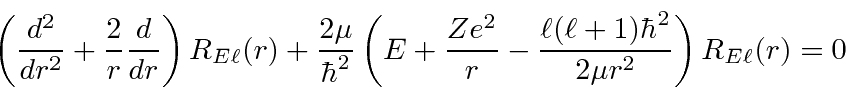
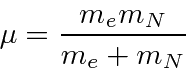
The differential equation can be solved using techniques similar to those used to solve the 1D harmonic oscillator equation. We find the eigen-energies
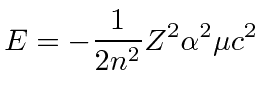 |
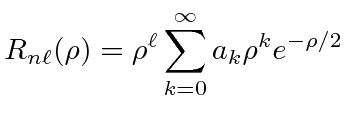 |
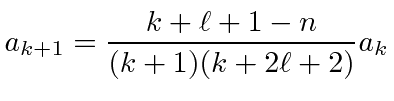 |
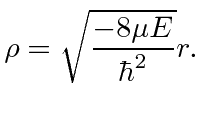 |
The principle quantum number
![]() is an integer from 1 to infinity.
is an integer from 1 to infinity.
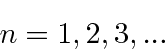
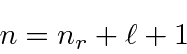
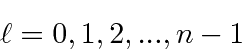
This unusual way of labeling the states comes about because a radial excitation has the same energy as an angular excitation for Hydrogen. This is often referred to as an accidental degeneracy.