Next: The Flux of Probability Up: The Schrödinger Equation Previous: The Schrödinger Equation Contents
For a free particle, we have
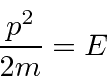
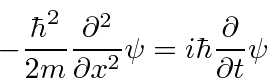
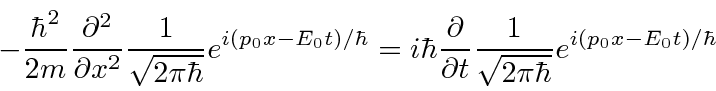
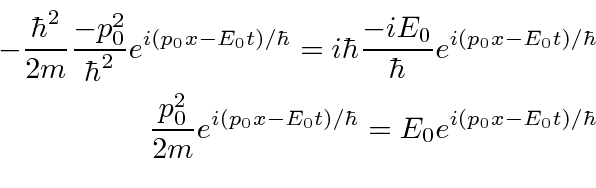
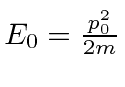 .
This is exactly what we wanted.
So we have constructed an equation that has the expected wave-functions as solutions.
It is a wave equation based on the total energy.
.
This is exactly what we wanted.
So we have constructed an equation that has the expected wave-functions as solutions.
It is a wave equation based on the total energy.
Adding in potential energy, we have the Schrödinger Equation
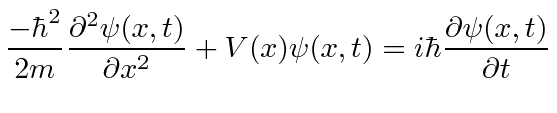 |
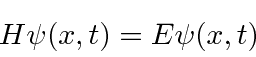
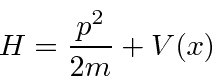
In three dimensions, this becomes.
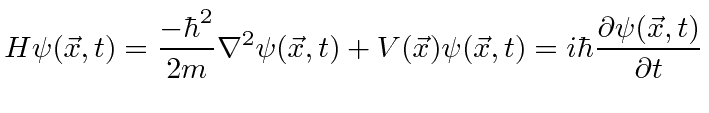 |
So the Schrödinger Equation is, in some sense, simply the statement (in operators) that the kinetic energy plus the potential energy equals the total energy.
Jim Branson 2013-04-22